Description
This course is a series of NGSS-aligned activities that present conceptual information in the context of a real-world scenarios and incorporate virtual lab activities throughout. The research reported here was supported by: (i) The Institute of Education Sciences, U.S. Department of Education, through Grants R305A100069, R305A170049 to WestEd. The opinions expressed are those of the authors and do not represent views of the Institute or the U.S. Department of Education. (ii) The National Science Foundation through Grant 1726856 to Carnegie Mellon University.
Descriptions of Activities
PowderAde
Students explore concentration through colored sports drinks. In the virtual lab, they prepare drinks with differing concentrations and use a spectrometer, which measures color, to determine the concentration of colored particles. They explore conversions and think proportionally and molecularly about the ingredients within PowderAde.

Making IV Solutions
Students explore dilution and concentration by making patient IV solutions. They learn about the molecular structure of salt and glucose and connect the microscopic to what is measurable. Students use proportional reasoning to obtain the desired volume and molarity of solutions in the virtual lab.
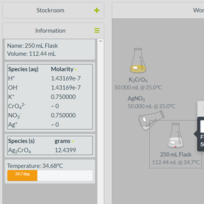
Gravimetric Analysis
Through a combination of particulate-level representations and virtual lab activities, students learn how gravimetric analysis can be used to determine the concentration of various species in water. Students review precipitation reactions and use the virtual lab to find out which chemicals react to form precipitates. Using stoichiometry to connect mass to concentration, students determine if the water is safe to drink based on EPA guidelines.

Bioremediation
Getting bacteria to eat oil is a powerful approach to cleaning up oil spills, and the first step is adding a bioremediation accelerator to form clumps that the bacteria will eat. Students perform experiments to determine the stoichiometric relationship between the accelerator molecules and the oil molecules so they can recommend the correct amount of accelerator. The reaction between the remediator and the oil provides a context for understanding stoichiometric proportions and limiting reagents.
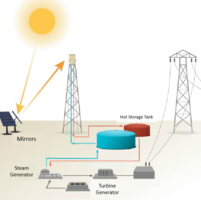
Solar Plant
This activity covers temperature, heat transfer, and heat capacity in the context of renewable and sustainable energy. The setting is a solar plant that uses thousands of mirrors to reflect sunlight onto the top of a tower, where the thermal energy from the sun is transferred to a liquid. The liquid then flows into an electric plant where the stored thermal energy is converted to electrical power. By designing and testing experiments, students consider heat capacity and boiling point as they establish the best substance for storing and transferring thermal energy.
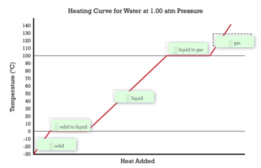
Phase Changes
In the context of phase changes, students infer the strength of electrical forces within and between particles. Students conduct an investigation of vapor pressure, comparing the macroscopic, earth's water cycle, to the microscopic, the intermolecular forces of water and other gases in the atmosphere. Students learn about the heating curve model and the relationship between temperature and kinetic energy.

Hot/Cold Pack
In this activity, students explore heat transfer and temperature by considering the design of hot and cold packs used to treat minor injuries. In the virtual lab, students evaluate substances by comparing their change in temperature when added to water. Students investigate kinetic energy at the molecular level and connect heat transfer to transfer of kinetic energy between systems. Throughout, students learn that endothermic and exothermic reactions are linked to the design of hot and cold packs.

Equilibrium
This activity uses color-changing reactions to explore chemical equilibrium, including the nature of reversible reactions and the use of LeChatlier’s principle. Students investigate the concentration of reactants and products, connecting these to the balance of forward and reverse reaction rates at equilibrium.